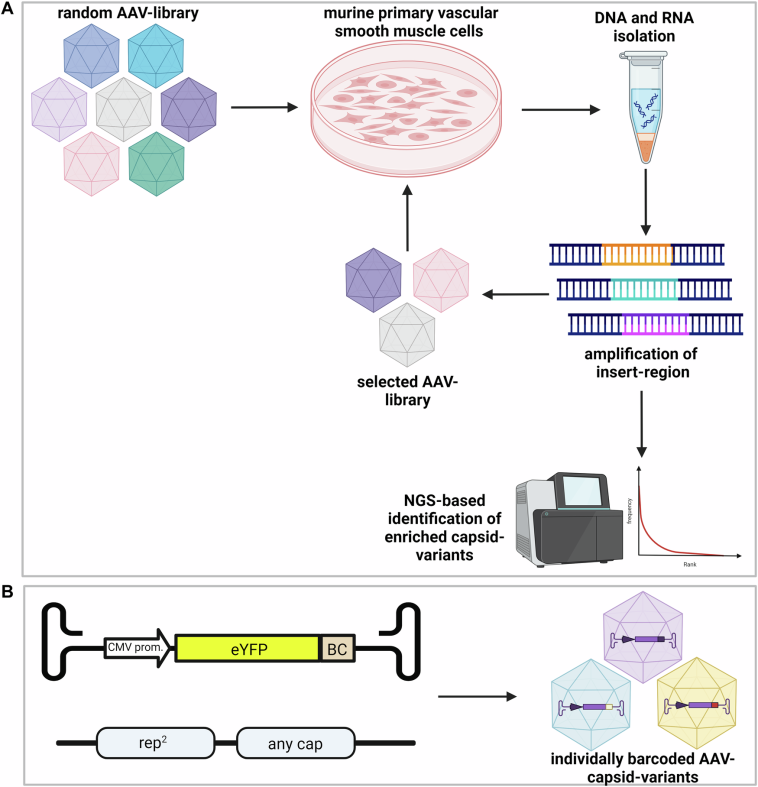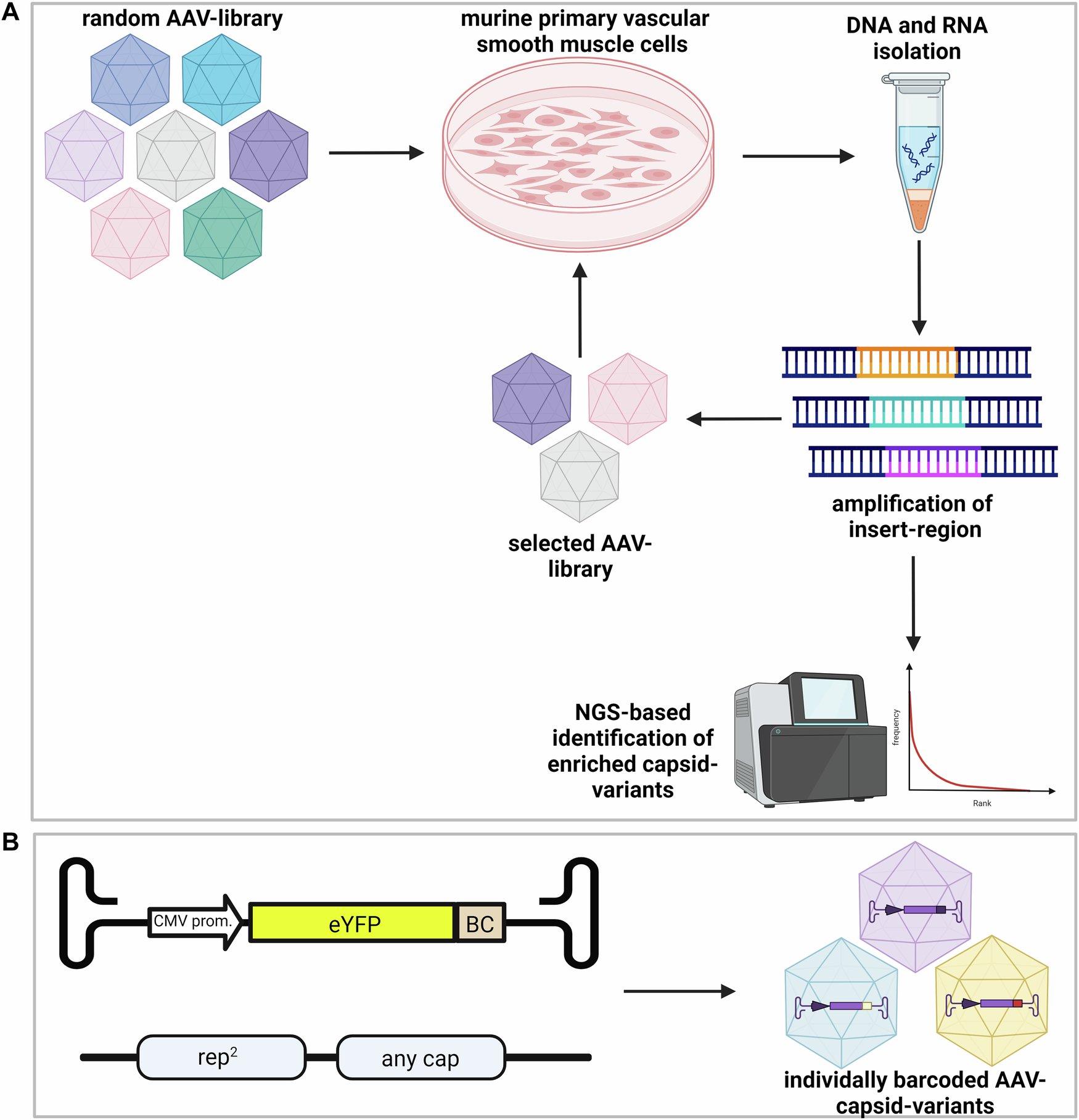
New Horizons in Gene Therapy: AAV Vectors and Vascular Health
Recent advances in gene therapy, particularly those involving adeno-associated virus (AAV) vectors, have stirred excitement and cautious optimism among researchers and clinicians. A groundbreaking study led by Maria Stamataki has showcased how reengineering AAV capsid proteins can dramatically enhance the delivery of therapeutic genes into human vascular endothelial cells. This progress offers hope for patients with vascular diseases and outlines a new path for treating conditions like atherosclerosis and thrombosis with greater precision and fewer side effects.
The study’s findings are illuminating, especially for those of us trying to figure a path through the tricky parts of optimizing gene therapy techniques. By harnessing the natural affinity of AAV vectors and modifying their structure, the research team managed to achieve significantly improved transduction efficiency – meaning the virus could deliver genetic material into cells at levels far superior to what traditional methods have achieved.
Refining AAV Engineering: Tackling the Troublesome Pieces
Gene therapy is not without its challenges, and optimizing gene delivery vectors remains one of the most complicated pieces of the puzzle. In this study, the critical focus was placed on tweaking the AAV capsid proteins. These outer proteins play a decisive role in determining which cells the vector can “home in” on, a factor that is absolutely key when targeting specialized tissues like vascular endothelial cells.
The Role of AAV Capsid Proteins in Vascular Targeting
The AAV’s capsid acts as the delivery vehicle. By reengineering the structure of these proteins, scientists can modify their ability to penetrate the endothelial barrier that lines blood vessels. This modification helps the virus sidestep some of the tricky parts of cellular delivery that have historically made gene therapy feel like a nerve-racking venture.
Consider these crucial steps taken by the research team:
- Screening a comprehensive library of AAV capsid variants in non-human primate models.
- Evaluating the performance of each variant in terms of cell penetration and transgene expression.
- Identifying variants that achieved over ten times more gene expression compared to traditional AAV serotypes.
These steps help clear some of the tangled issues and confusing bits associated with conventional gene therapy methods. By fine-tuning the AAV’s capsid proteins, the researchers have set the stage for a new era in gene delivery that offers promise for vascular diseases.
Screening in Non-Human Primates: Insights from Preclinical Studies
The use of animal models, particularly non-human primates, in preclinical screening, plays an essential role in this type of research. Despite the nerve-racking concerns regarding how well animal models translate to human outcomes, these tests offer a necessary glimpse into the in vivo performance of novel therapeutic vectors.
Diving Into the Benefits of Animal Models
Non-human primates provide a more accurate reflection of the human biological system than do smaller animal models, such as mice or rats. When working through the tricky parts of ensuring a new gene therapy works safely and effectively, researchers must rely on these models to:
- Assess the transduction efficiency of modified AAV variants in a living organism.
- Monitor how well the virus can target and enter vascular endothelial cells.
- Evaluate the safety profile and immune response generated by these advanced vectors.
Despite the complexities inherent to working with animal models, their use remains a critical, if intimidating, part of the process. They help scientists navigate through the fine points of dosing, viral load, and potential side effects, ensuring that only the most promising therapeutic strategies move forward.
Optimizing Dosing and Safety in Gene Therapy
One of the most nerve-racking challenges in gene therapy is determining the optimal viral load. The study’s extensive dose-response evaluations are a shining example of how researchers can manage their way through the dose optimization labyrinth. Not only did these studies assess gene expression levels, but they also looked for any signs of adverse reactions, ensuring that the modified AAV variants are both effective and safe for eventual clinical use.
Key Considerations in Dosing Studies
When planning any gene therapy protocol, several factors must be taken into account to balance efficacy with safety:
- Transgene Expression Levels: Achieving a high level of gene expression is critical to therapeutic success, yet overexpression can sometimes lead to unwanted side effects.
- Immune Response: The modified AAV vectors need to maintain a low profile so as not to trigger an overwhelming immune reaction, which could jeopardize treatment.
- Long-Term Efficacy: It is important to optimize the vectors so that they provide a sustained therapeutic effect without repeated administrations.
This nuanced approach not only ensures that the gene therapy is potent but also that it is sustainable over the long term. In essence, the research team is taking a measured approach to figure out a path that minimizes risks while maximizing the treatment’s benefits to patients.
Targeting Vascular Endothelial Cells: A Closer Look at the Fine Points
Vascular endothelial cells, which make up the interior lining of blood vessels, are critical targets in the fight against cardiovascular diseases. Their role in maintaining vascular health, as well as being involved in pathological processes like inflammation and plaque buildup, makes them super important when it comes to therapeutic gene delivery.
Tackling the Tricky Challenge of Cell-Specific Delivery
One of the major goals of this research was to overcome the convoluted pieces around cell-specific transduction. Traditional AAV vectors, while generally safe, have struggled to consistently and efficiently deliver genes into endothelial cells. However, by engineering AAVs that are more “endothelium-friendly,” the research team managed to achieve a breakthrough that could revolutionize how we treat a myriad of vascular conditions.
Key benefits of targeting vascular endothelial cells include:
- Enhanced Therapeutic Precision: Delivering the correct gene to the right cell type ensures that the therapeutic benefits are maximized.
- Reduced Side Effects: By limiting off-target transduction, the risk of undesirable effects in other tissues is minimized.
- Potential in Multiple Conditions: Improved gene delivery to vascular cells offers new therapeutic avenues for diseases like coronary artery disease, peripheral artery disease, and even stroke.
This innovative approach is gradually pushing the envelope of what gene therapy can achieve. With cardiovascular conditions on the rise globally, these advancements could lead to super important breakthroughs that change the way we approach treatment for vascular diseases.
Engineering AAV Capsids: Overcoming the Nitty-Gritty of Viral Vector Modification
The process of engineering AAV capsids is far from straightforward. It involves a deep dive into the subtle parts of viral structure and function – the nitty-gritty details that determine how efficiently a virus can enter a cell. Often, these modifications are a series of trial and error experiments, combined with advanced next-generation sequencing techniques that let researchers poke around the performance of hundreds of variants at once.
Key Techniques in Capsid Engineering
Several modern techniques have aided researchers in their quest to improve AAV performance:
- Next-Generation Sequencing (NGS): NGS provides a powerful tool to analyze the performance of various AAV variants in vivo, enabling the identification of those that perform best.
- Directed Evolution: This process involves generating a multitude of AAV variants and selecting the ones that show optimal traits for that particular application.
- Structural Biology Methods: Detailed imaging and structural analysis help in understanding how modifications to the capsid proteins affect cell targeting and immune evasion.
Using these approaches, scientists are able to overcome the tangled issues associated with the structural alterations of AAVs. Although it can be nerve-racking at times to work through the off-putting complexity of these modifications, the promise of a significantly improved gene delivery method is a powerful motivator.
The Promise of Enhanced Gene Therapy in Cardiovascular Diseases
Cardiovascular diseases have long been a major global health concern, and current treatment options often fall short, either due to limited efficacy or undesirable side effects. The leap forward represented by engineered AAV variants could offer a paradigm shift in not only treating but potentially curing a variety of vascular disorders.
Clinical Implications and Future Prospects
There are several key areas where enhanced gene therapy could have a transformative impact:
- Atherosclerosis Treatment: By delivering genes that can reduce plaque formation or even reverse existing plaque buildup, enhanced AAV vectors might offer a new line of defense against coronary artery disease.
- Thrombosis Prevention: Well-targeted gene therapy could modulate blood clotting factors directly within the vascular endothelium, reducing the risk of stroke and other thrombotic events.
- Regenerative Medicine: The potential to repair damaged blood vessels and promote tissue regeneration opens new avenues in treating ischemic conditions, where parts of the body receive insufficient blood flow.
Even though these applications are still many steps away from routine clinical use, the early results are promising. Moving forward, rigorous clinical trials will be essential to ensure both the long-term safety and the persuasive effectiveness of these engineered AAV vectors.
Addressing the Off-Putting Hurdles in Regulatory and Ethical Considerations
While technological advancements continue at pace, regulatory and ethical considerations remain loaded with problems that must be carefully managed. Ensuring that any new therapy is not only effective, but also safe over the long term, is super important both from a patient safety standpoint and from a regulatory perspective.
Considerations for Future Clinical Application
There are several key layers to work through when it comes to the clinical translation of these technologies:
- Long-Term Safety: Ensuring that the modified AAV does not induce undesirable immune responses or long-term adverse effects remains a critical piece of the puzzle.
- Ethical Approval: As with any emerging treatment, clinical trials must be conducted under strict ethical guidelines to protect patients and provide transparent results.
- Regulatory Oversight: Regulatory bodies such as the FDA and EMA must be satisfied that the improved transduction efficiency offered by engineered vectors does not come at the cost of patient safety.
As the research delves further into this topic, it is essential to balance the groundbreaking potential of these therapeutic vectors with a cautious and methodical approach to safety and ethics. Only through robust and transparent clinical testing can we ensure that this innovative technology is ultimately beneficial to patients.
Working Through the Potential Ripple Effects of Improved Gene Therapy
The implications of enhanced AAV-mediated gene therapy extend far beyond vascular diseases. The underlying technology of capsid engineering carries the promise of being adapted for multiple applications, ranging from neurological disorders to muscular dystrophies. With every new discovery, the potential therapeutic horizons widen, offering hope to patients across a broad spectrum of conditions.
Expanding Therapeutic Applications
In addition to addressing cardiovascular diseases, improved AAV vectors may also pave the way for breakthroughs in other medical areas. Some potential applications include:
- Neurological Disorders: Fine-tuned gene delivery systems might be developed to target specific neural cell types, opening possibilities for the treatment of conditions such as Parkinson’s disease and Alzheimer’s disease.
- Muscular Dystrophy: Optimizing gene transduction in muscle cells could offer new treatments for conditions like Duchenne muscular dystrophy, where there is a dire need for more effective therapeutic strategies.
- Ophthalmic Diseases: The eye, with its unique immune environment, represents another promising area for targeted gene therapy, particularly in conditions that affect retinal cells.
By broadening the scope of these therapeutic strategies, researchers can address multiple health challenges simultaneously. While each new application has its own set of tricky parts and subtle details, the advancements in capsid engineering provide a strong foundation to build upon.
Current Challenges and the Way Forward
Despite these promising outcomes, several challenges remain on the road to fully realizing the potential of enhanced AAV gene therapy. Each new study brings with it a series of interconnected issues that need to be resolved. Among these are:
- Scalability of Production: Producing engineered AAV vectors in quantities sufficient for widespread clinical deployment is a non-trivial challenge, requiring both technological innovation and significant investment in manufacturing infrastructure.
- Consistency of Results: As treatments become more personalized, ensuring that these advanced vectors consistently achieve the desired level of transduction in diverse patient populations stands as a critical, albeit intimidating, challenge.
- Cost Considerations: Balancing the high costs associated with gene therapy development and manufacturing against the potential benefits for patients is another issue that needs to be worked through by both scientific and business stakeholders.
In many ways, these challenges are like the maze of complicated pieces that researchers must navigate as they work toward safe, effective, and affordable gene therapy. However, the strides made so far inspire confidence that these hurdles can be overcome with the concerted effort of the global research community.
The Future Outlook: Optimism Amid Uncertainty
Looking ahead, the field of gene therapy appears poised on the brink of transformative breakthroughs. The discovery of AAV variants with enhanced transduction capabilities in vascular endothelial cells represents just one of many exciting developments that could redefine modern medicine. As we figure a path through the rough and tangled issues of gene therapy research, the potential applications become ever more enticing.
Bridging Laboratory Discoveries to Clinical Realities
A critical next step involves bridging the gap between laboratory research and effective clinical solutions. Key areas of focus will include:
- Scaling Preclinical Success: Extensive testing in larger animal models and eventual human clinical trials will be necessary to ensure that the promising results seen in early studies translate into real-world benefits.
- Long-Term Follow-Up Studies: Continuous monitoring after treatment will help determine the durability of gene expression and any delayed adverse effects, ensuring that these advanced therapies remain safe in the long run.
- Collaborative Research Efforts: The convergence of fields – from bioengineering and genetics to immunology and clinical medicine – will be essential in refining these novel vectors and tailoring them for various therapeutic applications.
As the investigation of these therapies continues, the scientific community must remain mindful of the small distinctions that arise throughout the process. Every minor tweak in engineering, every dosage adjustment, and every refinement in the delivery mechanism could play a critical role in how effectively these treatments work in practice.
Conclusion: A Promising Future for AAV-Mediated Gene Therapy
In summary, the recent advances in AAV vector engineering mark a significant leap forward in the art of gene therapy. By reworking the AAV capsid proteins, researchers have managed to overcome many of the tricky parts associated with delivering therapeutic genes into human vascular endothelial cells. The study led by Maria Stamataki provides robust evidence that targeted gene therapy can evolve from a nerve-racking, experimental approach into a streamlined, efficient treatment for cardiovascular diseases and beyond.
While challenges remain – from manufacturing scalability to long-term safety concerns – the optimistic outlook is well warranted. Through rigorous preclinical and clinical evaluation, the promise of improved AAV-mediated gene therapy could soon translate into real improvements in patient care.
As we take a closer look at the unfolding potential of this technology, it is evident that the evolution of gene therapy is filled with both promise and complexity. Navigating through the confusing bits and tangled issues of early research offers a glimpse of a future where genetic disorders and vascular diseases might be overcome by solutions that are not only innovative but also precisely tailored to individual patient needs.
The journey ahead may be fraught with challenges, but the path is steadily being cleared by innovative science and collaborative research efforts. As the field continues to progress, patients around the world could ultimately benefit from treatments that were once considered off-putting or overwhelmingly experimental. In the end, the fusion of biology and engineering in gene therapy exemplifies the spirit of modern medicine – a relentless pursuit of solutions to some of our most pressing health issues.
In this era of rapid advancement and continual rethinking of how best to approach health care, every subtle part refined in the laboratory moves us closer to therapies that are both safer and more effective. The story of AAV vectors and their evolving role in gene therapy is only beginning, and with it comes a new chapter in the treatment of cardiovascular and other critical diseases. The importance of this development cannot be overstated, and it serves as a beacon of hope for the future of medicine.
Ultimately, as we make our way through the twists and turns of scientific discovery and clinical application, the lessons learned from these pioneering studies will have far-reaching implications. With each step forward, the potential to alleviate human suffering grows ever more tangible. The work behind engineering these improved AAV variants is a testament to the power of collaboration, perseverance, and the scientific method – a true reflection of the promise that gene therapy holds for generations to come.
As researchers dive in and continue to refine these methods, the medical community and patients alike can look forward to a future where genetic conditions are managed more effectively, and where advanced therapies bring relief to those burdened by longstanding vascular and other diseases. The horizon is bright, and the journey, though challenging, is well worth the effort to achieve breakthroughs that will redefine how we treat some of the most stubborn health challenges of our time.
Originally Post From https://bioengineer.org/aav-variant-discovery-boosts-human-vascular-cell-transduction/
Read more about this topic at
Adeno-associated virus as a delivery vector for gene …
Optimized dual-AAV base editor delivery system with …